- Request a quote
 972–73-2126119
972–73-2126119

Scheme design:
DNA samples were sent out to the participating laboratories according to the diseases and mutations reported to be tested by them. In most cases, two samples were provided for each gene/disease for diagnosis, by the method routinely employed by each participating laboratory.
Reporting requirements:
Performance Criteria:
Consultants:
| Disease | Gene | Mutations |
| Mucolipidosis Type IV (ML4) | MCOLN1 | c.-1015_789del |
| c.1207C>T | ||
| Spinal Muscular Atrophy (SMA) | SMN1 | Exons 7-8 del |
|
Tay-Sachs Disease |
HEXA |
c.509G>A |
| c.1274_1277dup | ||
| c.571-2A>G | ||
| Joubert Syndrome Type II | TMEM216 | c.218G>T |
| Bloom Syndrome | BLM | c.2207_2212delinsTAGATTC |
| Walker Warburg Syndrome | FKTN | c.1167dup |
| Infantile Cerebral Cerebellar Atrophy -ICCA | MED17 | c.1112T>C |
| Familial Mediterranean Fever (FMF) | MEFV | c.1105C>T |
| Achondroplasia | FGFR3 | c.1138G>A |
| Deafness, Autosomal Recessive 7 | TMC1 | c.1939T>C |
| c.1810C>T | ||
| c.1210T>C | ||
| Progressive Cerebello-Cerebral Atrophy Pontocerebellar Hypoplasia type 2E (PCCA2) | VPS53 | c.1556+5G>A |
| c.2084A>G | ||
| Breast and Ovarian Cancer | BRCA2 | c.7007G>C |
| Colon cancer | MUTYH | c.1187G>A |
| Cockayne type B | ERCC6 | c.1034_1035insT |
|
Albinism |
OCA2 |
c.79G>A |
| c.1441G>A | ||
| c.79G>A | ||
| c. 1320G>C | ||
| c. 2373_2375del | ||
| Albinism | TYR | c.1A>G |
| c.1118C>A | ||
| MELAS-Mitochondrial disease | MT-TL1 | m.3243A>G |
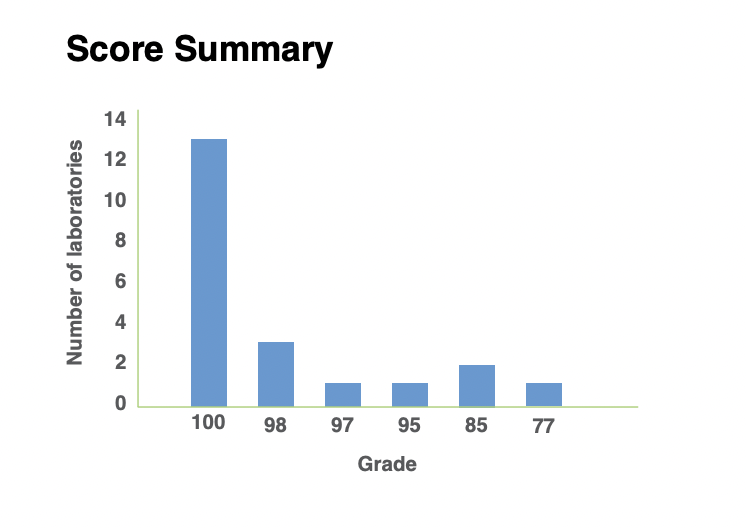
Score summary
Number of samples sent …………………….258
Number of diseases tested ………………….24
Genotyping errors………………………………4
Samples mix-ups……………………………………..0
Errors in nomenclature…………………………….3
Deficiencies in the final results report……..2